As the oxidation state of Na is 1 and Hydrogen is 1 because here hydrogen exists as a hydride. Summaiyafatima60 is waiting for your help.

Find Oxidation Number Nah204 A Nabh4 Chemistry Organic Chemistry Some Basic Principles And Techniques 13496312 Meritnation Com
A NaH2PO4 b NaHSO4 c H4P2O7 d K2MnO4 e CaO2 f NaBH4 g H2S2O7.
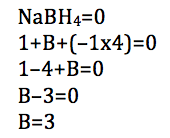
Oxidation no. of b in nabh4. Let the oxidation state of Boron be X. Mohit Gupta answered this. 2 For N a B H 4.
11062018 The key difference between LiAlH4 and NaBH4 is that LiAlH4 can reduce esters amides and carboxylic acids whereas NaBH4 cannot reduce them. These observations are consistent with our experimental data. Hence the oxidation state of boron is 3.
Two practical sources of hydride-like reactivity are the complex metal hydrides lithium aluminium hydride LiAlH 4 and sodium borohydride NaBH 4. The reaction of pure ultrafine Co2B and a pure sample of NaB02 at 500 C carried out as a control yielded metallic cobalt 500 oc ol nlo Co2B N023H20 - COS B20 - 2h. 19072011 Subsequent analysis found that it consisted of Na 2 B 4 O 7 10H 2 O unidentified B x O y and B 2 O 3.
X41 1 x 14 x 3 S o oxidation number of B in N aBH 4. 20160107 by Haseeb Mohammed. These are both white or near white solids which are prepared from lithium or sodium hydrides by reaction with aluminum or boron halides and esters.
Properties of hydride sources. 05012021 Calculate the Oxidation number of underlined elementaNaBH4 bCr2O7. But LiAlH4 is a very strong reducing agent than NaBH4 because the Al-H bond in the LiAlH4 is weaker than the B-H bond in NaBH4.
What are the oxidation states in Borohydride BH4- The CRC Elecrochemical Series and the solution to our exercise suggest that the oxidation states of borohydride are -V for Boron and I for Hydrogen. Add your answer and earn points. Oxidations were carried out using 30 hydrogen peroxideNaOH after the addition of methanol 2 ml and THF 20 ml following the reported procedure 6.
As the oxidation state of Na is 1 and Hydrogen is -1 because here hydrogen exist as hydride. Assign oxidation number to the underlined elements in each of the following species. N a BH 4.
Answer verified by Toppr. The high activities of the NaBH 4 - and NH 3 BH 3 -reduced Co catalysts can. C Na2B4O7 sodium Borate.
Many of us remember traces of oxidation and reduction from general chemistry tossing around electrons on balanced equations LEO GER or OIL RIG to help us remember which is whichIn. Hence the oxidation state of boron is 3. 23092018 oxidation no of Na is 1 since oxidation no of alkali metal is 1 oxidation no of H is -1 hydrogen in hydrides will have -1 sum of oxidation numbers in a compound is 0 so let oxidation no of boron be x 1x 4 -10 solving it we get x3 oxidation no of B is 3.
Both LiAlH4 and NaBH4 are reducing agents. 08082008 Boron B atom number 5 the 1ST member of group 13 has an oxidation state of 3 it is rather a metaloid even more than Al 2nd member so it forms an acidic oxide B2O3. Since H 22 is more electronegativ than B 20 I would have expected the oxidation states to be III for B and -I for H.
CThe crude product obtained on oxidation with diethyl etherchromic acid system 7 gave 2-decanone in 4 yield. Oxidation no of Na is 1 since oxidation no of alkali metal is 1 oxidation no of H is -1 hydrogen in hydrides will have -1 sum of oxidation numbers in a compound is 0 so let oxidation no of boron be x 1x 4 -10 solving it we get x3 oxidation no of B is 3. B H3BO3 boric Acid.
B after heat treatment at 500 C for 120 min under argon. Let the oxidation state of Boron be X As the oxidation state of Na is 1 and Hydrogen is -1 because here hydrogen exist as hydride. Give The Oxidation Number Of Boron In Each Of The Following.
BYields are of the products isolated after distilation. What is the oxidation number of B in NaBH4. Therefore X 1 4 -1 0 X 3 Hence the oxidation state of boron is 3.
Calculate the Oxidation number of underlined element. X 1 4 -1 0. 22112013 Mohit Gupta Meritnation Expert added an answer on 241113.
Formed by the controlled oxidation of ultrafine CaB followed by water wash a as received. Give The Oxidation Number Of Boron In Each Of The Following. 30012013 When students start getting to the chapters on alcohols and carbonyl groups aldehydes ketones etc they come across the important process of oxidation and reduction.
Why Does Boron Have A 3 Oxidation State Quora
No comments