Ammonium sulphate has the chemical formula NH 4 2 SO 4. The formula for hydrogen gas is H 2.
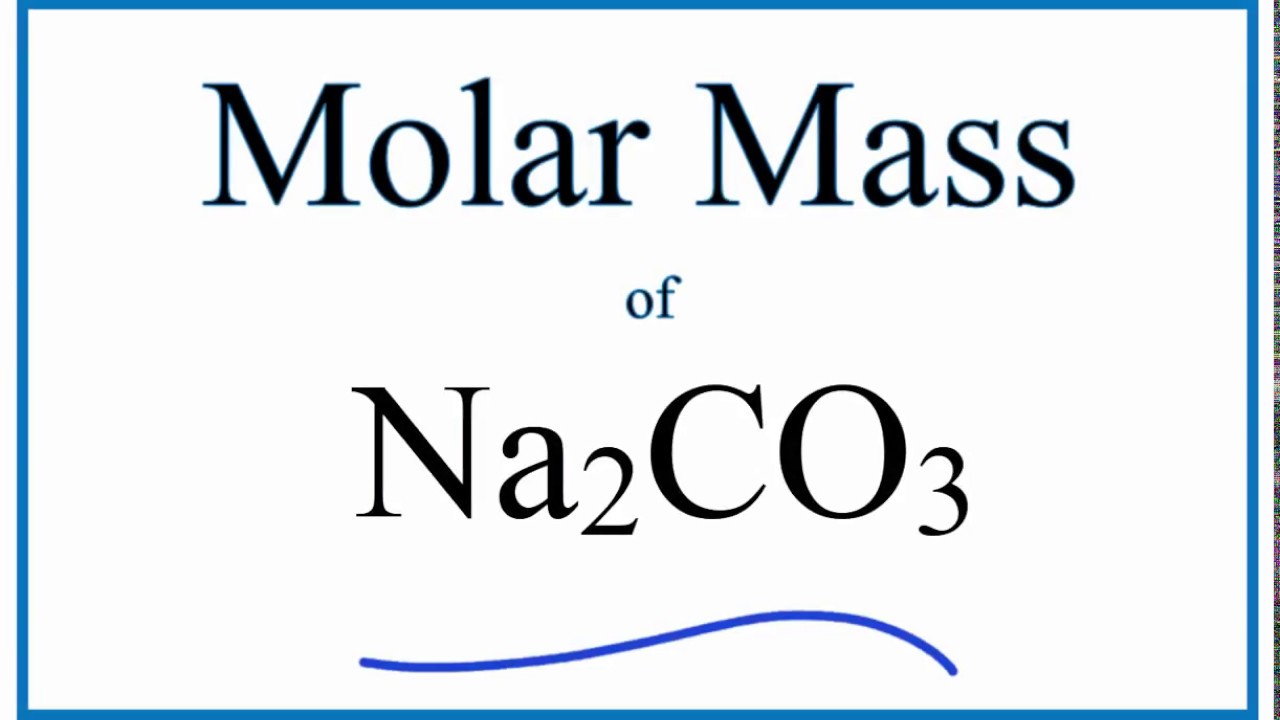
Molar Mass Molecular Weight Of Na2co3 Sodium Carbonate Youtube
If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight.

Calculate the relative molecular mass of nahco3. Examples of molecular weight computations. H 1 Solution. To calculate the molar mass of a compound multiply the subscript of each element from the formula times the molar mass of the element.
The molar mass of any compound is given by the sum of the standard atomic weight of the atoms which form the compound. 2020-02-14 Beside above what is the mass in grams of 094 moles of sodium bicarbonate NaHCO3. Also to know is how do you calculate percent by mass.
What is the relative mass formula of hydrogen gas. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. Asked by ABHILASHA 24th Feb 2018 0241.
2018-02-24 Calculate molecular mass of sodium bicarbonate. For a single sample the relative atomic mass of the sample is the weighted arithmetic mean of the masses of the individual atoms present in the sample also known as the average atomic mass. 2 x 14 28.
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Queries asked on Sunday. Sodium bicarbonate NaHCO3 is often ingested at a dose of 03 gkg body mass BM but ingestion protocols are inconsistent in terms of using solution or capsules ingestion period combining NaHCO3 with sodium citrate Na3C6H5O7 and coingested food and fluid.
It is soluble in water. 1 u is equal to 112 the mass of one atom of carbon-12. Answered by Varsha 24th Feb 2018 0451.
Mahnasimah mahnasimah 04062019 Chemistry Secondary School answered Calculate Molecular mass of NaHCO3. How to calculate the relative formula mass. 8 x 1 8.
Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Then mass n x molar mass 094 840 gmole 8796 g. 40x 1 40 C x 1.
Molecular formula of sodium bicarbonate is NaHCO3 Molecular weight of sodium bicarbonate is 2311248 84. 4 x 16 64. The purpose of the study was to quantify the effect of ingesting 03 gkg NaHCO3 on blood pH.
The molar mass of an element is its atomic weight relative atomic mass on the periodic table in. Examples of molecular weight computations. Definitions of molecular mass molecular weight molar mass and molar weight.
840057 gmol 1g119039541364455E-02 mol Percent composition by mass. Calculate the relative molecular mass of CaCO3. Element Count Atom Mass by mass.
See similar Chemistry GCSE. Calculate the moles of NaHCO3 in the sample and record the data in the data tablemass69206g. The relative mass formula of hydrogen gas is M r H 2.
Relative atomic mass Ar. The relative atomic mass of a compound is the ratio of the average mass of the elements in a chemical compound to the atomic mass constant which is defined as 112 the mass of a carbon 12 atom. Molecular mass molecular weight is the mass.
ChemistryOne to one online tuition can be a great way to brush up on. C 12 Ca 40 O 16 Number of atoms Ca x1. Need help with.
12x1 12 O x 3 16 x 3 48CaCO3 RMM 40 12 48 100. If the substance is made of simple molecules this mass may also be called the relative molecular mass. Definitions of molecular mass molecular weight molar mass and molar weight.
Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter its formula specify its isotope mass number after each element in square brackets. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight.
4 x 16 64. After 7pm from Monday to Saturday will be answered. 840066 NaHCO3 is a white powder at room temperature.
Answered by Roxanne M. Each molecule contains 2 hydrogen atoms. Here we have multiplied the mass of a single NH 4 by two in the calculation on the left because there are.
In case of mathNaHCO_3math Sodium Bicarbonate the atoms that form the compound are Na H C and O. Calculate Molecular mass of NaHCO3 Get the answers you need now. 2 x 14 4 x 1 36.
Calculate the relative molecular mass of ammonium sulphate.

Pin By Michelle M On Knowledge Molar Mass How To Calculate Moles Teaching Chemistry

No comments